Can Pharmacist Change Date On C2 Prescription
Published July xx, 2010
Electronic Controlled Substances Prescriptions
US Pharm. 2010;35(7):65-68.
The Drug Enforcement Administration (DEA) has released its Interim Final Rule on Electronic Prescriptions for Controlled Substances With Request for Annotate, which was published in the Federal Register on March 31, 2010, and went into effect on June ane, 2010.one-iv According to the DEA, "The rule revises DEA regulations to provide practitioners with the choice of writing prescriptions for controlled substances electronically. The regulations also allow pharmacies to receive, dispense, and archive these electronic prescriptions. These regulations are an add-on to, not a replacement of, the existing rules. The regulations provide pharmacies, hospitals, and practitioners with the ability to use modern engineering for controlled substance prescriptions while maintaining the airtight system of controls on controlled substances."5,6
The Basics
In that location are some important caveats that pharmacists should note. The receipt and dispensing of controlled substances by means of electronic advice is optional. If electronic prescriptions are accepted by a pharmacy, the dominion allows the electronic archiving of prescriptions, subject to security and retrievability provisions. According to the DEA, "Persons who wish to prescribe/dispense controlled substances using electronic prescriptions must select software that meets the requirements of this rule. Awarding [emphasis added]7 providers who make such electronic prescribing software or pharmacy software available may wish to carefully review the requirements of this rule if they wish their software to handle electronic prescriptions for controlled substance. Every bit of June 1, 2010, simply those electronic prescription applications and pharmacy applications that comply with all of DEA'due south requirements as set forth in 21 CFR Part 1311 may be used by DEA-registered prescribing practitioners and DEA-registered pharmacies."8
Existing Country and Federal Laws
All other statutes and regulations that apply to the dispensing of controlled substances remain in consequence; the new provisions only broaden the existing mandates. For example, practitioners may notwithstanding communicate Schedule III, IV, and Five prescriptions by phone, by fax, or in written course.9 The big alter is that now Schedule 2 prescriptions may be communicated electronically in add-on to the traditionally required hardcopy written format. Retrieve that all land laws regarding the prescribing and dispensing of controlled substances remain in outcome; this federal rule does not preempt existing inconsistent or different state-based laws, although many states have been poised to allow electronic prescribing of controlled substances for some time now.10 Just remember that the stricter law applies.
Technology Requirements
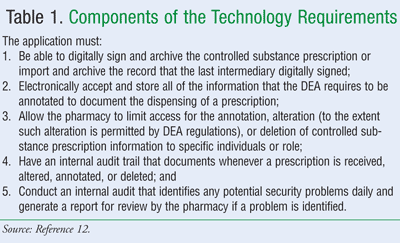
Earlier showtime to utilize the technology necessary to comply with the DEA regulations, a chemist's shop must accept in identify hardware and software that meets minimum security measures.11 Generally, the technology application must exist able to import, brandish, and shop the required contents of a controlled substance prescription accurately and consistently.12 In that location are several components to this requirement (meet TABLE 1). Some of these procedures are already standard measures for pharmacy operations and should not pose a hardship or crave massive new programs to achieve compliance mandates. However, information technology is the DEA-registered pharmacy that is responsible for ensuring whatsoever system is used meets all of the standards before electronic prescriptions for controlled substances are accepted nether the new rule.
Assurances
The are two alternatives for obtaining this assurance. 1 is to hire a 3rd party to perform an audit on the application to determine that each of the standards is met. The other is to have the application certified by a DEA-approved certification organization. Either way, the entity hired to do the system analysis volition outcome a report that states whether the application complies with the DEA's requirements and whether in that location are any limitations on its use for controlled substance prescriptions. There volition no incertitude be some electronic prescriptions that will crave revisions earlier they can exist accustomed electronically. If the report indicates that the pharmacy application cannot accurately and consistently import, store, and display necessary data, the pharmacy will not be allowed procedure electronic prescriptions for controlled substances. The DEA offers the example of hospital prescriptions issued to staff members with an identifying suffix. If the audit or certification report indicates that the pharmacy application cannot import, brandish, and store both a hospital DEA number and the private practitioner's extension number, the pharmacy will not be permitted to have electronic prescriptions that include but a infirmary DEA registration. The pharmacy may, however, utilize the application to process other controlled substance prescriptions if the audit or certification study has found that the pharmacy application meets all other requirements.
Preliminary Reports
The technology provider must give a copy of the study to pharmacies that use or are considering use of its application to allow a chemist's to determine whether the application is compliant with the DEA's requirements. Note that the pharmacy cannot accept electronic prescriptions and use them equally authority to manipulate controlled substances until the inspect or certification written report is issued and the chemist's has a written re-create of the application provider's approving of compliance with the DEA regulations in paw.
Limited Admission
Another mandate is that the pharmacy must limit access to the technology that records the receipt, processing, and dispensing data for electronically prescribed controlled substances medication orders. The access control may be set to apply individual names or by roles, such as pharmacist, pharmacy intern, or chemist's shop technician. This control measure must too define who has permission to annotate, alter (where amending is permitted by DEA regulations), or delete controlled substance prescription information.
Intermediaries
As indicated in TABLE i, you must exist able to digitally sign and archive controlled substance prescriptions or import and archive the records that the last intermediary digitally signed. As used in this regulation, intermediary ways any technology system vendor that receives and transmits an electronic prescription betwixt the practitioner and the chemist's. As a bones principle, understand that an electronic prescription must be transmitted from the practitioner to the chemist's in its electronic form. If an intermediary cannot transmit the electronic data file to the pharmacy, the intermediary must notify the prescriber. In this type of circumstance, if the prescription is for a Schedule Three, IV, or V controlled substance, the prescriber would have to print the prescription, manually sign it, and fax the prescription directly to the chemist's. This prescription must indicate that it was originally transmitted to, and provide the proper noun of, a specific pharmacy, the engagement and time of transmission, and the fact that the electronic transmission failed. Schedule II prescriptions would have to be issued in the traditional hardcopy format.
Manual
During normal electronic transmission between a prescriber and a pharmacy, the contents of a prescription cannot be altered. Note that this mandate applies just to the content; the electronic format of the prescription may vary depending on the intermediary's technological software. Note also that this variance applies but to intermediaries and not to changes made on a prescription later on a prescription is received in a pharmacy. The aforementioned laws and regulations that utilise to paper prescriptions govern changes made by the pharmacy.
Changes After Receipt of an Electronic Prescription
Exactly what changes can be made to the face of a Schedule 2 prescription, whether transmitted electronically or hand delivered to a chemist's, has been a source of confusion since the DEA issued its Final Rule, Issuance of Multiple Prescriptions for Schedule 2 Controlled Substances, in November 2007.xiii,14 In the rule's preamble, the DEA stated that "the essential elements of the [schedule II] prescription written by the practitioner (such as the proper name of the controlled substance, strength, dosage course, and quantity prescribed)…may non exist modified orally." This is in opposition to the DEA's previous policy, which permitted changes to Schedules III, IV, and V controlled substance prescriptions later on oral consultation with the prescriber. In a letter to the New Mexico Regulation and Licensing Section Board of Pharmacy in Jan 2010, the DEA acknowledged that interpreting this position to mean that "absent the power to make oral changes, a Schedule II prescription must be returned to the prescribing practitioner for correction or reissuance" creates a significant issue that they are planning to resolve through future rulemaking.15 In the meantime, the DEA instructs pharmacists "to adhere to state regulations or policy regarding those changes that a pharmacist may make to a Schedule II prescription subsequently oral consultation with the prescriber."sixteen As a outcome, some states, such as Kansas, have brash patients and pharmacists that a pharmacy cannot make whatsoever changes to the face up of a Schedule II prescription, or else "the pharmacy is subject area to subject field from the federal government."17
The regulations for making changes to Schedules III, Iv, and V prescriptions have not changed. Pharmacists may add together or change the patient's address upon verification, and change the dosage form, drug strength, drug quantity, directions for use, or issue engagement only afterward consultation with the prescribing practitioner; this must and so be noted on the prescription. The patient'southward name, prescriber's signature, and the drug prescribed (except for generic substitution permitted by state law) cannot be inverse. Whatever state or local laws prohibiting changes to prescriptions for controlled substances must be followed.18
Identity Proofing Prescribers
Earlier dispensing a controlled substance prescription, a pharmacist needs to exist sure that the prescriber has the proper credentialing to appoint in this practice. The following information may be useful to pharmacists in educating prescribers about the steps necessary to comply with electronic prescribing. The Rule requires prescribers to undergo an identity proofing procedure before being allowed to prescribe and transmit controlled substances electronically. According to the DEA, "identity proofing is critical to the security of electronic prescribing of controlled substances."nineteen The regulations land that authentication credentials used to sign controlled substance prescriptions may be issued just to DEA-registered prescribers (in person or by remote identity proofing) whose identity has been confirmed after applying and receiving credentialing from federally approved credential service providers (CSPs) or certification authorities (CAs) to obtain their two-cistron authentication credential or digital certificate. In achieving this standard, the CSP or CA will be required to deport identity proofing that meets National Institute of Standards and Technology Special Publication 800-63-1 Balls Level 3.
The DEA advises that information technology expects application providers will piece of work with CSPs or CAs to direct prescribers to one or more sources of 2-factor hallmark credentials that will be interoperable with their applications and that prescribers should contact their application provider to determine which CSP or CA the provider recommends that the prescriber use.20 Afterwards the identity proofing procedure is completed, the CSP or CA is required to either issue a new difficult token or register and provide credentials for an existing token. Communications between the CSP or CA and the approved prescriber must occur through two channels (e.g., post, phone, or e-mail).
2-Gene Credentialing
Insofar as the two-factor credentialing requirement goes, the DEA will accept knowledge (something unique that the prescriber knows), a hard token (stored somewhere other than the calculator being used for electronic prescribing), or a biometric identification (e.thousand., fingerprints). Hard tokens are cryptographic keys stored on a PDA, jail cell phone, smart-card, USB memory stick, or a one-fourth dimension utilize countersign device and are tangible, concrete objects possessed by the private prescriber. The credentialing information must be retained simply by the prescriber and not shared by any other person. Failure to secure the credentialing information or devices could expose the prescriber to suspension or revocation of a DEA registration.
The prescriber volition use the 2-factor credentials to digitally sign and archive prescriptions. Each controlled substance prescription must exist individually signed using the electronic signature method; multiple controlled substance prescriptions are not allowed using simply one signature. Issuing multiple prescriptions for different patients using just one signature is also prohibited.
It is important for pharmacists to sympathize that according to the rule, signing and transmitting an electronic controlled substance prescription are considered two distinct actions. While the DEA states that electronic prescriptions for controlled substances should be transmitted equally before long as possible after signing, information technology is understood that prescribers may prefer to sign prescriptions before office staff add together chemist's shop or insurance data. Therefore, the DEA is not requiring that transmission of the prescription occur simultaneously with signing the prescription.
Pharmacists may check the prescribers' certificates that permit electronic prescribing and the status of DEA registration with the DEA. Pharmacists should also keep in mind that prescribers must be licensed or otherwise authorized to prescribe controlled substances by the state where the act of prescribing is done.
Simultaneous Electronic and Hardcopy Prescriptions
The DEA anticipates that in that location may be instances where a prescriber electronically transmits a controlled substance prescription and also issues a newspaper prescription. In such an instance, the pharmacist is required to check the pharmacy records to ensure that the electronic version was not received and the prescription was not dispensed. If both prescriptions were received, the pharmacist must mark 1 every bit void. The newspaper prescription must comply with all DEA requirements for any newspaper prescription, including a manual signature.
There likewise could be times when an electronic prescription is originally transmitted to a dissimilar chemist's and the patient presents a newspaper prescription to your pharmacy. In this circumstance, the pharmacist must check with the other chemist's shop to make up one's mind whether the prescription was received and dispensed. If the other pharmacy received the original electronic prescription, but had not dispensed the prescription, that pharmacy must mark the electronic version as void or canceled. If the chemist's that received the original electronic prescription dispensed the prescription, the pharmacy with the paper version is not allowed to dispense medication using the newspaper prescription as authority and must mark the prescription as void.
Records
One time a prescription is created electronically, all records of the prescription must exist retained electronically. These records must be kept for a minimum period of 2 years (state laws usually have longer retention requirements). In addition, pharmacy application service providers must fill-in files daily. Although it is not required, the DEA recommends that pharmacies store their backup copies at another location to prevent the loss of the records in the outcome of natural disasters, fires, or system failures.
Security Failures
The pharmacy's system is required to run an internal audit for potential security incidents daily and generate a written report of any problems. If the software generates a study and, upon investigation, the person designated to administrate access controls for the pharmacy determines that the issuance or records of controlled substance prescriptions has been compromised or could have been compromised, the pharmacy must be reported to the application provider and DEA within one business 24-hour interval. In general, the security incidents that should be reported are those that correspond successful attacks on the application or other incidents in which someone gains unauthorized access.
Affect
How the prescribing community responds to the new rule is non anticipated. "But time will tell if the system is crushing to physicians and pharmacists," said Del Konnor, president of DEA Solutions Group, a consulting firm that advises health intendance organizations on complying with laws and regulations related to controlled substances. Mr. Konnor said he hopes DEA investigators volition apply the rule equally a "teaching aid initially to assist physicians and pharmacists better sympathize the intent of the new regulation rather than begin to use it to seek out technical violations."21
Surescripts, which operates the nation's largest east-prescribing network, said in a statement that the company plans to work closely with "members of the nation'due south pharmacies, chemist's benefit managers, health plans, software vendors, and prescribers to analyze and human activity on the DEA's requirements." The company said the DEA rule "may impose particular challenges for prescribers and pharmacies," but added that it plans to collaborate on solutions that let the prescribing community comply with the regulation'south standards and protocols. Co-ordinate to Surescripts data, past the end of 2009, 18% of eligible prescriptions were prescribed electronically (compared to 6.vi% in 2008).22
Summary
The above but addresses the basic components of the procedures mandated by the new rule on prescribing and dispensing electronic controlled substances prescriptions. Pharmacists should have the time to familiarize themselves with the details and become comfortable and competent in knowing and following all of the applicative regulations. Hopefully, once the systems are fully implemented the numbers of prescribing and dispensing errors will subtract along with incidences of preventable controlled substances diversion.
REFERENCES
i. Docket No. DEA-218, RIN 1117-AA61. 21 CFR § 1311, subpart C. Electronic prescriptions for controlled substances. www.admission.gpo.gov/su_docs/
2. 75 FR 16236 No. 61. Electronic prescriptions for controlled substances; final rule. March 31, 2010. www.deadiversion.usdoj.gov/
3. Caverly MW. Chief Liaison and Policy Section, Function of Diversion Control, USDOJ, DEA. Letter to electronic application providers and pharmacy application providers. Apr 2, 2010. world wide web.deadiversion.usdoj.gov/
4. Electronic prescriptions for controlled substances. world wide web.deadiversion.usdoj.gov/
v. Encounter Note 4, supra.
6. Rules—2010. Electronic prescriptions for controlled substances. www.deadiversion.usdoj.gov/
7. Equally perhaps only a government hierarchy can do with such supremacy, the DEA chose to utilize the word "awarding" to refer to the software and hardware engineering that makes electronic prescribing possible within the context of the security provision required by the DEA. When reading the regulations or DEA literature nigh the regulations, "application" could easily be misinterpreted to mean "the human activity of applying," equally in "submit an application" for permission to utilize a particular device. Clearly, the DEA is using the discussion as "the deed of putting something to a special employ or purpose." Consult any dictionary for further clarification.
eight. Encounter Note 3, supra.
9. Economic touch analysis of the acting final electronic prescription rule. March 2010. www.deadiversion.usdoj.gov/
10. 73 FR 36722. Electronic prescriptions for controlled substance; proposed rule. June 27, 2008. www.deadiversion.usdoj.gov/
11. 21 CFR §§ 1304.06(c), equally amended, and 1311.205.
12. Electronic prescriptions for controlled substances. Interim final dominion with asking for comment, questions and answers for pharmacies [as of 03/31/2010]. www.deadiversion.usdoj.gov/
xiii. Questions & answers. What changes may a pharmacist make to a prescription written for a controlled substance in schedule II? 72 FR 64921. www.deadiversion.usdoj.gov/
14. Meet 21 CFR §§ 1306.05 and 1306.11.
15. Letter from Marker Caverly, Chief Liaison and Policy Section, Role of Diversion Control, USDOJ, DEA, to Larry Loring, State Drug Inspector, New Mexico Regulation and Licensing Department, Boards and Commissions Division-Pharmacy. Jan 6, 2010. www.rld.state.nm.us/pharmacy/. Accessed May half dozen, 2010.
16. Run into Annotation 13, supra.
17. Letter from Debra L. Billingsley, Executive Secretary, Kansas Lath of Chemist's, addressed to Kansas patients. April 12, 2010. www.kansas.gov/pharmacy/. Accessed May 6, 2010.
18. See Note 13, supra.
19. Electronic prescriptions for controlled substances. Interim final dominion with request for comment: questions and answers for prescribing practitioners [every bit of 03/31/2010]. www.deadiversion.usdoj.gov/
twenty. See Annotation 19, supra.
21. Moore J. A second await at the DEA eastward-prescribing dominion. Authorities Health Information technology. March 29, 2010. http://govhealthit.com/
22. See Note 21, supra.
To comment on this article, contact rdavidson@uspharmacist.com.
Source: https://www.uspharmacist.com/article/electronic-controlled-substances-prescriptions#:~:text=Pharmacists%20may%20add%20or%20change,be%20noted%20on%20the%20prescription.
Posted by: cooperhavine.blogspot.com


0 Response to "Can Pharmacist Change Date On C2 Prescription"
Post a Comment